
PrimeStore® MTM is a molecular transport media that gives the user a “snapshot” in time for that sample by preserving and stabilizing DNA and RNA.
PrimeStore MTM (Molecular Transport Medium) was designed and optimized for molecular testing allowing pathogenic samples to be collected, transported, and processed safely and efficiently. Millions of tubes of PrimeStore MTM have been sold to clients around the world during the SARS-CoV-2 pandemic.
The molecular transport medium inactivates infectious biological pathogens including viruses, and gram-positive/negative bacteria whilst preserving and stabilizing labile DNA and RNA for downstream molecular applications.
Viral transport media (VTM) were designed for transporting samples intact to be cultured prior to the widespread use of molecular testing and contain ingredients that inhibit optimal molecular testing. In contrast, PrimeStore MTM was invented and optimized specifically for molecular testing.
As the only FDA Class 2 cleared product for microbial nucleic acid storage and stabilization, hospitals and laboratories trust that PrimeStore MTM provides safer and more reliable testing than UTM/VTM, saline, or research use only devices.
Laboratories have independently validated PrimeStore MTM with a range of platforms such as Roche, Thermo Fisher, Abbott, Siemens, Luminex, Bio-Rad, PSS, Promega, and BioMerieux, as well as the Cepheid GeneXpert point of care platform.
A key feature of PrimeStore MTM is that it allows lab teams to process multiple tests from a single sample that has been inactivated and stabilized in PrimeStore MTM.
There are multiple peer-reviewed scientific papers, dating from 2011 onwards, supporting the use of PrimeStore MTM for pathogen detection from a wide range of clinical matrices and biofluid types collected and processed in different countries. A selection of these can be found below.
‘At the University of Leicester we have established an asymptomatic SARS-CoV-2 screening programme, providing reassurance and support to our staff and students on campus with the aim to reduce viral transmission.
By choosing PrimeStore Molecular Transport media with its viral inactivation properties, we are ensuring the safe transportation and processing of participants samples and ensuring protection for our staff. Furthermore, we have the reassurance of RNA stabilisation, protecting sample integrity at room temperature without the need for additional storage requirements. EKF have provided excellent customer service throughout. Offering a fast and friendly personalised approach, addressing all of our technical considerations and fully supporting our needs.’
Leicester University
“Our primary reason for choosing the PrimeStore MTM was for safety. The MTM inactivates the SARS-CoV-2 virus whilst stabilising the RNA. This means that we can safely handle samples we receive that could potentially contain a hazard group 3 pathogen in a Class II MSC. This in turn helps our workflow as it removes the need to inactivate the samples prior to moving to the next step.”
Yourgene Health
|
Organism |
Positive control (cfu/mL) |
PrimeStore MTM + Organism (% killed) |
|
E. coli |
6.4 x 107 |
100 |
|
S. aureus |
6.0 x 107 |
100 |
|
MRSA |
4.7 x 106 |
100 |
|
Organism |
Positive control (TCID50/mL) |
PrimeStore MTM + Organism (% killed) |
|
Influenza A (H3N2) |
7.5 x 108 |
>99.99 |
|
Adenovirus type 5 |
7.5 x 108 |
>99.99 |
|
Influenza A (H5N1) |
7.5 x 107 |
>99.99 |
*Initial 4-log dilutions of PrimeStore MTM + viruses were required due to PrimeStore lysis of tissue culture.
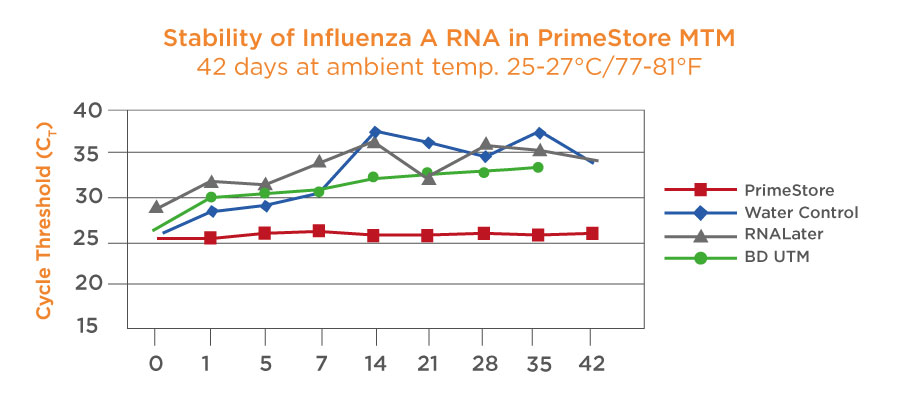
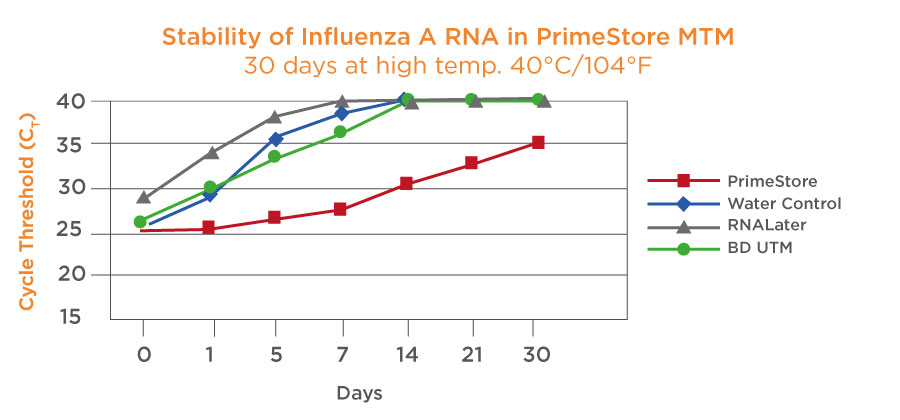
Unlike most sample collection systems PrimeStore MTM alleviates cold chain requirements for sample collection and transportation, ensuring RNA stability for 7 days at ambient temperatures or 28 days at 2-8C.
The PrimeStore MTM collection tube is optimised for short, medium- and long-term storage as samples placed in PrimeStore MTM are ready for biobanking with no requirements for additional supplies.
PrimeStore MTM safely inactivates pathogenic samples whilst preserving DNA and RNA by denaturing nucleases and proteases. This allows sample processing to be performed outside of controlled containment and eliminates the risks associated with transporting live pathogenic samples.
|
Manual spin column kits |
Automated magnetic bead extraction kits |
|
PrimeXtract™, Longhorn Vaccines and Diagnostics |
EZ1®, Qiagen |
|
RNAqueous®-Micro Kit, Thermo Fisher Scientific |
QIAsymphony® Virus/ Bacteria Mini Kit, Qiagen |
|
QIAamp® Viral RNA and DNA Mini Kits, Qiagen |
NucliSENS® easyMAG®, BioMerieux |
|
ChargeSwitch® Total RNA Cell Kit (Invitrogen), Thermo Fisher Scientific |
MagNA Pure 96 System DNA and Viral NA Small Volume Kit, Roche |
|
MagMAX™ Viral RNA Isolation kit (Ambion), Thermo Fisher Scientific |
PrimeBeads™, Longhorn Vaccines and Diagnostics |
Please note that the user is responsible for validating downstream extraction and purification methodologies. PrimeStore MTM should not be used with any analysers that utilise a bleach decontamination step such as Hologic platforms. Platforms that do not have a nucleic acid extraction step should not be used in the final assay as PrimeStore MTM will denature all enzymes used within the assay.
Flocked swabs of man-made materials (i.e. not cotton with a wooden shaft) can be used. At this time Longhorn does not have experience with 3D printed swabs in PrimeStore MTM.
The procedure is specific to the extraction kit being used and not specific to PrimeStore MTM. Samples must be incubated in PrimeStore for 60 minutes prior to DNA/RNA extraction.
The sample should be collected and placed into the PrimeStore MTM solution, it should be noted that samples (including swabs) should be added at a maximum ratio of one part sample to three parts PrimeStore MTM.
If using a sample swab, the swab should be snapped at the breakage point and then sealed into the PrimeStore MTM vial.
The user should then shake the vial for 3-5 seconds and allow the sample to incubate in PrimeStore MTM for a minimum of 60 minutes prior to sample processing. Samples should be vortexed for 3-5 seconds prior to DNA and RNA extraction.
Labs processing samples collected in PrimeStore MTM should handle all waste by following their own Waste Disposal Protocols for their routine nucleic acid extraction kits (after acid).
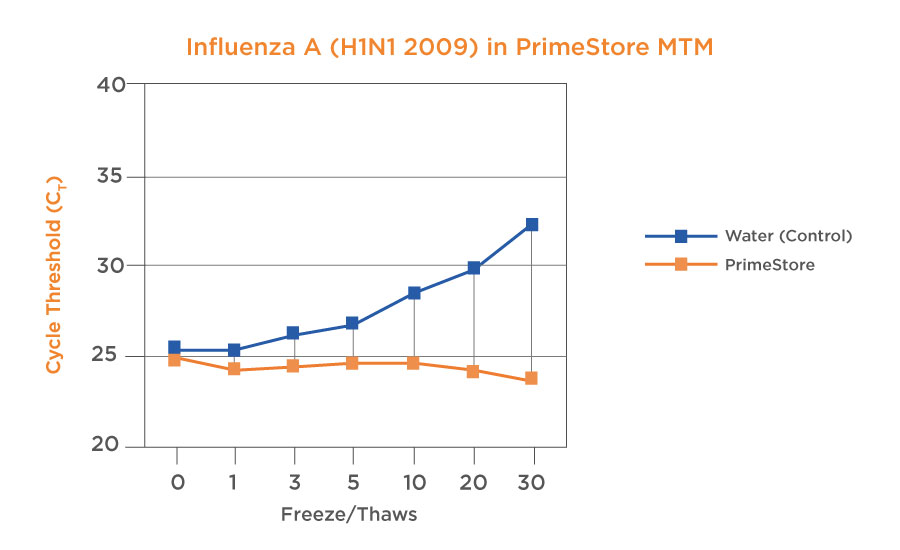
Samples are Stable for 7 days at ambient temperature and stable for 28 days at 2-8oC. Samples can be bio-banked for long term storage. It should be noted that samples will not be affected by multiple freeze-thaw cycles. Studies have shown longer stability for RNA at both ambient and high temperatures from time of collection to time of nucleic acid extraction compared with other transport media.
Due to certain components of the PrimeStore MTM solution, you must first undertake a nucleic acid extraction process before you conduct a RT-PCR assay. However you can place a sample from PrimeStore MTM into the Cepheid GeneXPert cartridge along with the Cepheid PBS solution without first performing an extraction step.
Copyright © 2024 | Powered by Intergage

Please note: Not all products are available in all countries. Please check availability.
Newsletter
Keep up to date with all our activities, events, exhibitions, promotions, investor news & more.